Post Market Survaillance Program
Iradimed Post-Market Surveillance program is designed to be proactive & data-driven to ensure quality, compliance, and patient safety throughout the entire lifecycle of our medical devices.
Dashboards
Program Visuals & Key Areas
Complaint Handling & Reporting
Fig. 1.1 Complaint handling and medical device reporting consists of more than just timely filing adverse event reports.
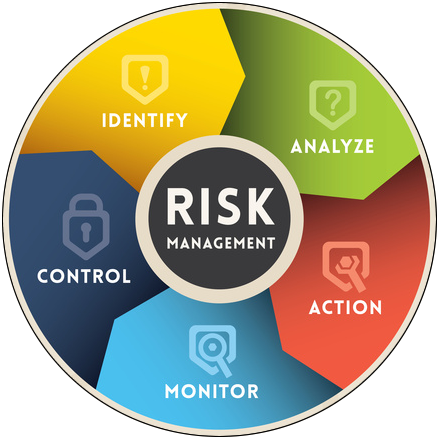
Risk Management Integration
Fig. 1.2 Risk management and complaint handling are closely intertwined within an organization's overall quality management system.

Data Analytics & Visualizations
Fig. 1.3 From Complaints to Action: Using Trends and Statistics to Drive Meaningful Change
Our Process Flow
Intake & Triage
Feedback Gathered
Is it a complaint?
No
Trend Data
Yes
Proceed to Investigation
Investigation & Action
Evaluate & Investigate
Adverse Event?
Yes
Report to Authorities
No
CAPA Required?
Yes
Initiate CAPA
No
Analysis & Reporting
Aggregate & Analyze Data
Update Risk Management File
Generate Periodic Reports
(PSUR, PMCF)
(PSUR, PMCF)